We started off this week with learning about organic molecules like lipids, proteins, carbohydrates and nucleic acids. Later in the week we discussed macromolecules and biochemistry. On Thursday we made cheese and eggs!
Lipids store energy, and include hormones, cholesterol, and phospholipids. Lipids are made up of their carboxyl group, a hydrocarbon chain, which resembles a chain of carbon surround by hydrogen, and a hydroxide ion. When the fatty acid is unsaturated there is a double bond in this hydrocarbon chain.
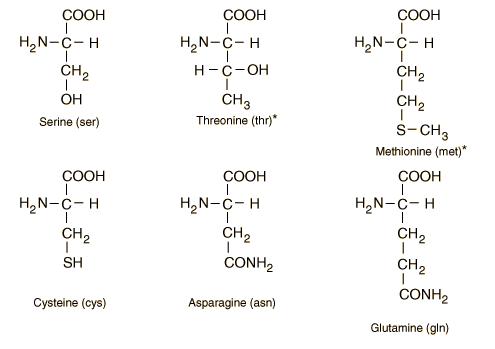
Overall, there are 20 different types of amino acids. Amino acids are comprised of an amine group (NH3), a carboxyl group (-COOH), and an ‘R’ group. The R group determines an amino acid’s identity (4.A.2). Every amino acid is made up of Nitrogen bonded to carbon, which is bonded to another carbon.
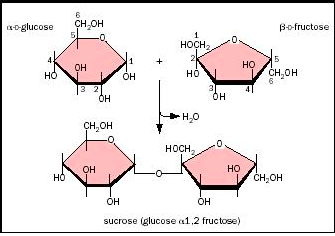
Carbohydrates are the body’s energy carriers. Carbohydrates are made up of glucose, chain sugars, and a ring of polysaccharides. We learned about mono and disaccharides. When two monosaccharides bond into order to make a disaccharide, they release a water molecule and bond at an oxygen atom, a bond known as a dehydration bond.
Nucleotides are made up of a phosphate group, a sugar, and a nitrogenous base. The nitrogenous bases are also know as adenine, guanine, cytosine, thymine, and uracil (3.A.4).How would a nucleic acid react to a bigger phosphate group? And what would happen is a disaccharide was used instead of a monosaccharide?
The last day of the week we had some fun with milk and eggs! So, milk is actually a mixture of fat and protein in an aqueous solution and caesin, the protein in milk, is extremely hydrophobic and can be easily denatured by changes in pH and the addition of high temperatures.
How it changes by pH:
The H+ floating around in the solution as a result of the addition of acid will try to bond with parts of the amino acids and by doing so it breaks some of the intramolecular bonds that give the molecule its shape, causing it to straighten out into a long chain. However this does not change the polarity of the molecule and the molecule try get away from the water. What would happen if we introduced a base instead rather than an acid?
How it changes by heat: by adding heat we add more energy to the individual atoms. What would happen if we removed some heat or did not heat it up as much? Would that stabilize the amino acid?
(THANKS MR. DUNN FOR THE AMAZING PANCAKES)




This was a great summary of the past 2 weeks. Adding a base, with the knowledge I have currently from Mr. Dunn, would not do anything without more energy.
LikeLike